 Facilitatori: un nuovo approccio alla terapia genetica
Facilitatori: un nuovo approccio alla terapia genetica
sono una nuova prospettiva per il trattamento di diverse malattie legate ad una scarsa o difettosa produzione genica. Essi agiscono intervenendo sullo specifico programma cellulare. Un sistema di regolazione che agisce a diversi livelli (trascrizione, traduzione, post-traduzione). Ne sono manifestazioni i cambiamenti della cromatina, la trascrizione, il processamento dell’RNA, il trasporto dell’RNA al citoplasma, la regolazione post trascrizionale (microRNA), la traduzione, il taglio proteolitico e le modificazioni chimiche delle proteine, la degradazione delle proteine. I ricercatori hanno trovato delle molecole che possono agire in tutte queste fasi riattivando/modulando processi genetici spenti/ipo- od iperattivi.
Applicazioni
La terapia scoperta potrebbe già essere attiva in alcune malattie diffuse nel bacino del Mediterraneo come la thalassemia o l’anemia falciforme. Molte altre malattie ematiche potrebbero però trarne giovamento.
Researchers have found a promising new method for gene therapy according to a new research from the Hubrecht Institute (De Laat group), Erasmus MC and Sanquin, published in the journal Blood.
The rearchers successfully restarted inactive genes by bringing them closer to genetic switches on the DNA called enhancers. The intermediate piece of DNA was cut out using CRISPR-Cas9 technology. This strategy opens up new possibilities for treating genetic diseases. The team specifically shows the technology’s potential for the treatment of sickle cell disease and beta-thalassemia, two genetic blood diseases. In these conditions, a faulty gene could potentially be compensated by reactivating a helpful but normally inactive one. This ‘delete-to-recruit’ method works by simply changing the spacing—without adding new genes or foreign elements.
The genes in our DNA carry instructions for making proteins, which perform a range of functions in our cells. But not all genes are active at all times. For instance, some proteins are only needed when specific nutrients need to be broken down. Others only carry out functions during embryonic development and are inactive later in life. For our cells to function properly, it is therefore important that gene activity is precisely regulated.
One way to do this is through enhancers: stretches of DNA capable of turning genes on, like a genetic switch.
Bringing it closer
Enhancers can be located next to the gene they control, but can also be far away on the DNA. “In this study, we discovered that it’s possible to activate a gene by bringing it closer to an enhancer,” says Anna-Karina Felder, one of the study’s first authors. Felder and her colleagues Sjoerd Tjalsma, Han Verhagen and Rezin Majied achieved this by using CRISPR-Cas9, a technology acting as molecular scissors that can be guided very precisely to cut the DNA. “We directed the scissors to cut out a piece of DNA between an enhancer and its gene, bringing them closer together,” Felder explains. “In adult cells, this successfully reactivated genes that are normally only active during embryonic development”. The team refers to this entirely new way of reactivating genes as ‘delete-to-recruit’.
Faulty hemoglobin
The new strategy offers hope for patients with sickle cell disease and beta-thalassemia. In these genetic blood diseases, the adult globin gene is broken. This causes the protein hemoglobin, responsible for carrying oxygen in our red blood cells, to not form properly. As a result, red blood cells are broken down too quickly and patients suffer serious lifelong symptoms such as anemia, fatigue and, eventually, organ damage. Blood transfusions are often necessary.
Restarting the backup engine
Delete-to-recruit technology could be used to treat these patients by harnessing the fetal globin gene. This gene is naturally active before birth, and part of the hemoglobin produced within the fetus. Once the child is born, it is switched off. “In people with sickle cell disease or beta-thalassemia, it’s the adult globin gene—the main engine that powers red blood cells—that is broken. But fetal globin is like a backup engine. By switching it back on, we can repower the red blood cells and possibly cure these patients,” Felder, one of the main author of research, said.
New possibilities
“While we’re still in the early stages, this research lays important groundwork for the development of new gene therapies,” Felder says. This goes beyond the scope of genetic blood diseases, as the new method could also be applied to other diseases where insufficient amounts of healthy proteins can be compensated by restarting a ‘backup engine gene’. The broader field of gene therapy could thus benefit from delete-to-recruit technology, because it uses a different approach than currently available therapies.
di Guido Francesco Guida

 Pubblicato un nuovo studio peer -reviewed su Nutrition Research condotto alla Oregon State University (USA) che dimostra i benefici dell’assunzione di mandorle nella sindrome metabolica detta anche sindrome X, da insulino-resistenza, o di Reaven.
Pubblicato un nuovo studio peer -reviewed su Nutrition Research condotto alla Oregon State University (USA) che dimostra i benefici dell’assunzione di mandorle nella sindrome metabolica detta anche sindrome X, da insulino-resistenza, o di Reaven.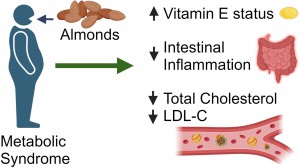 La sindrome metabolica è una condizione clinica caratterizzata dalla presenza di un cluster (aggregato) tipico delle nostre società. Coesistono infatti obesità centrale, dislipidemia, resistenza all’insulina, ipertensione arteriosa e disfunzione endoteliale (rivestimento interno dei vasi). Una molteplicità di fattori che, fra loro correlati, concorrono ad aumentare la possibilità di sviluppare diabete, malattie a carico dell’apparato cardio-circolatorio e, recentemente, disfunzione cognitiva e demenza.
La sindrome metabolica è una condizione clinica caratterizzata dalla presenza di un cluster (aggregato) tipico delle nostre società. Coesistono infatti obesità centrale, dislipidemia, resistenza all’insulina, ipertensione arteriosa e disfunzione endoteliale (rivestimento interno dei vasi). Una molteplicità di fattori che, fra loro correlati, concorrono ad aumentare la possibilità di sviluppare diabete, malattie a carico dell’apparato cardio-circolatorio e, recentemente, disfunzione cognitiva e demenza. Gli effetti benefici delle mandorle sono noti da tempo. Esse, infatti, per la loro alta concentrazione di grassi monoinsaturi e polinsaturi, riducono i livelli di colesterolo LDL (cosiddetto cattivo), l’infiammazione e la sensibilità all’insulina. Inoltre, possono svolgere un ruolo nella gestione del peso in quanto la loro densità nutrizionale e il contenuto di fibre e proteine favoriscono la sazietà, aiutando così a ridurre l’apporto calorico complessivo (Dreher et al., 2017). Mandorle come spuntino inoltre migliorano la tolleranza all’esercizio fisico e riducono la percezione del dolore muscolare nei soggetti moderatamente in sovrappeso (Crisp et al., 2020).
Gli effetti benefici delle mandorle sono noti da tempo. Esse, infatti, per la loro alta concentrazione di grassi monoinsaturi e polinsaturi, riducono i livelli di colesterolo LDL (cosiddetto cattivo), l’infiammazione e la sensibilità all’insulina. Inoltre, possono svolgere un ruolo nella gestione del peso in quanto la loro densità nutrizionale e il contenuto di fibre e proteine favoriscono la sazietà, aiutando così a ridurre l’apporto calorico complessivo (Dreher et al., 2017). Mandorle come spuntino inoltre migliorano la tolleranza all’esercizio fisico e riducono la percezione del dolore muscolare nei soggetti moderatamente in sovrappeso (Crisp et al., 2020).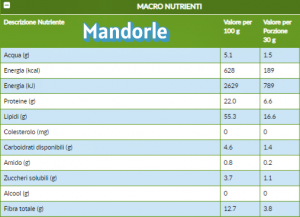
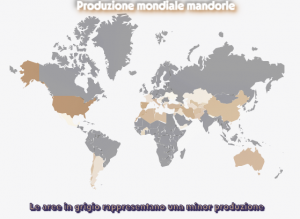 La mappa dei Paesi produttori mostra poi un dato alquanto suggestivo: ad una ridotta produzione corrisponde una minore aspettativa di vita.
La mappa dei Paesi produttori mostra poi un dato alquanto suggestivo: ad una ridotta produzione corrisponde una minore aspettativa di vita. “A tutti raccomando il rispettar la dieta … e chi lei non apprezza, quando sano mal regge e infermo non ben si cura” Questo già intorno all’anno 1000” era scritto nel Regimen Sanitatis Salernitanum della Scuola Medica Salernitana.
“A tutti raccomando il rispettar la dieta … e chi lei non apprezza, quando sano mal regge e infermo non ben si cura” Questo già intorno all’anno 1000” era scritto nel Regimen Sanitatis Salernitanum della Scuola Medica Salernitana. La prima, di un certo rilievo, venne realizzata nel 1992. Da allora se ne sono succedute diverse versioni fino a quella proposta oggi che presenta:
La prima, di un certo rilievo, venne realizzata nel 1992. Da allora se ne sono succedute diverse versioni fino a quella proposta oggi che presenta:
 Ma la soluzione era ben diversa: occorreva intervenire profondamente nei modelli gestionali alla ricerca di una medicina personalizzata, più organizzata e competente. Più vicina ai cittadini i quali, da parte loro, dovrebbero partecipare attivamente con comportamenti sia di prevenzione che di cura consapevole ed attiva. E’ un cambio di paradigma: non più “ospedalocentrico”, ma di integrazione ospedale-territorio. Dove “un nuovo territorio” previene e tratta la maggior parte delle malattie e riserva agli ospedali, dialoganti con esso, i casi acuti e particolarmente complessi. Si sono quindi cercati modelli gestionali innovativi che potessero agire da filtro efficiente agli ospedali ridistribuendo l’impegno sanitario e garantendo una migliore soddisfazione ed efficienza dei servizi socio-sanitari.
Ma la soluzione era ben diversa: occorreva intervenire profondamente nei modelli gestionali alla ricerca di una medicina personalizzata, più organizzata e competente. Più vicina ai cittadini i quali, da parte loro, dovrebbero partecipare attivamente con comportamenti sia di prevenzione che di cura consapevole ed attiva. E’ un cambio di paradigma: non più “ospedalocentrico”, ma di integrazione ospedale-territorio. Dove “un nuovo territorio” previene e tratta la maggior parte delle malattie e riserva agli ospedali, dialoganti con esso, i casi acuti e particolarmente complessi. Si sono quindi cercati modelli gestionali innovativi che potessero agire da filtro efficiente agli ospedali ridistribuendo l’impegno sanitario e garantendo una migliore soddisfazione ed efficienza dei servizi socio-sanitari. Come già detto elementi fondamentali per la realizzazione del nuovo progetto di assistenza saranno la piena attuazione del fascicolo Sanitario Elettronico e la telemedicina. Quest’ultima con finalità preventive diagnostiche terapeutiche e riabilitative. Sono previsti, nell’ambito del telecontrollo e teleconsulto: televisita, teleconsulenza medico-sanitaria, teleassistenza, telemonitoraggio.
Come già detto elementi fondamentali per la realizzazione del nuovo progetto di assistenza saranno la piena attuazione del fascicolo Sanitario Elettronico e la telemedicina. Quest’ultima con finalità preventive diagnostiche terapeutiche e riabilitative. Sono previsti, nell’ambito del telecontrollo e teleconsulto: televisita, teleconsulenza medico-sanitaria, teleassistenza, telemonitoraggio.
 ad una crisi della maggior parte di tali società scientifiche dovuta non solo alla recente pandemia da Covid-19, ma a ragioni pratiche e di sostanza. Crisi che si concretizza in una scarsa partecipazione alle occasionali iniziative societarie non solo di natura scientifica. E in una riduzione del numero degli iscritti attivi – spesso non noti – e che comunque fonti qualificate segnalano andare dal 50 al 60% ed, in taluni casi, spingersi fino all’80-90%. Le cause non possono essere attribuite solo a carenza di fondi, ma ragionevolmente possono essere catalogate, per chiarezza di esposizione, in esterne – in buona parte subite – ed interne, talora volute.
ad una crisi della maggior parte di tali società scientifiche dovuta non solo alla recente pandemia da Covid-19, ma a ragioni pratiche e di sostanza. Crisi che si concretizza in una scarsa partecipazione alle occasionali iniziative societarie non solo di natura scientifica. E in una riduzione del numero degli iscritti attivi – spesso non noti – e che comunque fonti qualificate segnalano andare dal 50 al 60% ed, in taluni casi, spingersi fino all’80-90%. Le cause non possono essere attribuite solo a carenza di fondi, ma ragionevolmente possono essere catalogate, per chiarezza di esposizione, in esterne – in buona parte subite – ed interne, talora volute.

 L’inquinamento causa problemi non solo ai polmoni, ma anche al cuore.
L’inquinamento causa problemi non solo ai polmoni, ma anche al cuore.
 ti alle radiofrequenze in utero hanno mostrato avere un peso leggermente più basso alla nascita. I risultati ottenuti sui topi non sono stati ancora resi noti. Pur essendo stata ripresa da diverse testate giornalistiche invero la notizia è una anticipazione poiché lo studio completo sarà pubblicato nel 2017. I tumori sviluppati sarebbero qulli in qualche modo già legati alle radiofrequenze e cioè i gliomi maligni nel cervello e gli schwannomi nel cuore dei ratti maschi esposti. Finora i risultati di studi condotti in questo settore sono stati piuttosto contrastanti. Infatti se da una parte alcuni studi europei (progetto europeo Interphone) ed australiani avevano escluso alcun tipo di rapporto causa effetto tra le microonde prodotte dai cellulari ed il cancro, l’Organizzazione mondiale della sanità nel 2011 aveva classificato, basandosi su alcuni studi in laboratorio e su ricerche epidemiologiche, le radiofrequenze nel gruppo 2b. Cioè come ‘possibili agenti cancerogeni’ per gli stessi tipi di tumori riportati dalla ricerca dell’NTP.
ti alle radiofrequenze in utero hanno mostrato avere un peso leggermente più basso alla nascita. I risultati ottenuti sui topi non sono stati ancora resi noti. Pur essendo stata ripresa da diverse testate giornalistiche invero la notizia è una anticipazione poiché lo studio completo sarà pubblicato nel 2017. I tumori sviluppati sarebbero qulli in qualche modo già legati alle radiofrequenze e cioè i gliomi maligni nel cervello e gli schwannomi nel cuore dei ratti maschi esposti. Finora i risultati di studi condotti in questo settore sono stati piuttosto contrastanti. Infatti se da una parte alcuni studi europei (progetto europeo Interphone) ed australiani avevano escluso alcun tipo di rapporto causa effetto tra le microonde prodotte dai cellulari ed il cancro, l’Organizzazione mondiale della sanità nel 2011 aveva classificato, basandosi su alcuni studi in laboratorio e su ricerche epidemiologiche, le radiofrequenze nel gruppo 2b. Cioè come ‘possibili agenti cancerogeni’ per gli stessi tipi di tumori riportati dalla ricerca dell’NTP.